The Program for Pandemic Preparedness by Ventilation, P3Venti, leads, collects and distributes scientific research on the influence and potential impact of ventilation on aerogenic pathogen spread, exposure risk and infection control.
P3Venti has an expected duration of three years (August 2022 – July 2025), is coordinated by TNO, and carried out by a consortium of research institutes and partners financed by the Dutch ministry of health (VWS).
For more in-depth information on the program, please refer to the program summary.
The goal of P3Venti is to support the government and (not-for-profit) stakeholders in their policy and decision making by generating scientific, actionable knowledge. Three key areas can be identified in this task:
Symposium P3Venti
Thursday the 9th of October 2025 the closing symposium of P3Venti will take place in Zoetermeer.
read moreScience
A critical part of P3Venti is fundamental scientific research. Scientific publications will be collected on a database on this website as they are published.
Our research aims to build knowledge in several key areas:
Research areas
Technical research
This research includes studying exposure risks and the effect of ventilation measures, observations in operational conditions such as elderly care homes, interviews with stakeholders and occupants, computer modeling and experimental particle dispersion and airflow vector research both in a mock-up and in-situ.
What contribution do ventilation and the use of air purifiers etc. make to preventing infection?
Influence of indoor environmental conditions, such as humidity and temperature.
Implementation and policy-making
Knowledge from the other two areas is collected and combined with social-scientific methods to better transfer the knowledge to, and facilitate the implementation of measures for the users.
Prioritization. In which sectors of society is (investing in) ventilation as a prevention measure most needed and most effective?
Proportionality and cost/benefit of application of ventilation.
Biomedical research
Investigation of viability and infectivity of virus-bearing particles (especially related to SARS-CoV-2) in relation to time and size distribution. Focus on literature studies and experimental research.
The contribution of aerogenic transmission to the total transmission of a virus.
The dose-response relationship in air, i.e. how many virus particles are needed to cause infection through aerogenic transmission?
Network
To ensure the accessibility and dissemination of the scientific findings and knowledge, P3Venti is establishing a knowledge network.
The core of the knowledge network are the members of a broad scientific sounding board, and the researchers within the program. From this core, the network will expand and grow as the program matures, facilitating partnerships and collaboration between related scientific programs and projects, such as PDPC, CLAIRE and MIST; as well as promoting collaboration and feedback with healthcare institutions and regulatory bodies.
With this network, P3Venti not only provides answers to the specific research questions defined for this program, but also contributes to the strengthening of the knowledge base needed to identify and effectively address new research questions and needs in a timely manner.
The network will increasingly expand to include delegated professionals connected to long-term care and possibly from other social sectors. As such, P3Venti is actively looking to make national and international connections with interested scientists and organizations to start exchanging knowledge and expertise. In this spirit, we would like to invite and encourage you to reach out and contact us if you have any interest in joining the Network by attending our symposium, joining our LinkedIn group, following the LinkedIn information page, or sending any questions to penvoerder-p3venti@tno.nl.
An initial overview of current key players within the knowledge network can be found on the network page.
Links:
Practice
Based on the knowledge gained and collected through P3Venti, we strive to provide practical measures, methods and guidelines to help healthcare institutions and regulatory bodies to be better prepared for the next pandemic. A vital aspect is to as much as possible ensure healthy and enjoyable living conditions under these measures. Here you can find questions, answers and tips on how ventilation and air purification can contribute to reducing the spread of viruses through the air.
Questions and answers
Effectiveness of ventilation and air purifiers
Can ventilation reduce the risk of infections?
Ventilation can reduce the concentration of aerosols (small airborne particles) – and therefore viruses - in an indoor environment. Implementing ventilation does not automatically reduce the number of infections throughout a building; the effect can vary significantly from one room to another. The effectiveness depends on many factors, such as the specifications of the ventilation system, relative humidity, room temperature, air distribution, airflow patterns, and the air exchange rate of the room.
Ventilation has little or no effect on infection risk when the aerosol emitter and the receiver (person) are in close proximity. In addition, air flow has negligible impact on the dispersal of very large particles (larger than 100 µm). Their dispersal is primarily dependent on gravitational effects. Ventilation primarily contributes to reducing the concentration particles that travel and spread over longer distances. Effectiveness of ventilation in reducing aerosol concentration is subject to a number of framework conditions.
Recirculation (air extracted from the indoor environment is pumped back into the indoor environment) of indoor air between rooms should be avoided whenever possible. Use of recirculating units within on room, for the purpose of (additional) heating and/or cooling, need not be a problem, as long as sufficient fresh air is supplied through ventilation. The presence of recirculating units will not exacerbate the spread of virus particles through the room, though it may accelerate the rate of dispersal through additional mixing. Strong air currents in the room should be avoided. These may significantly elongate the ‘exhalation plume’ containing virus particles emitted by the source. This may increase the likelihood of other persons in the room at distances exceeding 1.5m becoming infected.
Nowadays, systems recirculating air across multiple rooms are seldom encountered in Dutch buildings. Such systems as are still operational are almost exclusively located in older structures. Given an adequate supply of fresh air, the presence of recirculation is not necessarily problematic: dilution and particle removal rates will be adequate. In buildings that house relatively large numbers of infected individuals and/or highly vulnerable groups, it is best not to use these recirculation systems. In these cases it is better to use fresh air supply exclusively for ventilation.
Correct use of ventilation systems is an important prerequisite for ventilation effectiveness. If a ventilation system is not used or maintained properly, its theoretical performance specifications will not be realized in practice. The website www.ventilerenzogedaan.nl provides a range of tips for the correct use of ventilation systems. For more information, see the usage inventory of ventilation systems in Dutch long-term care facilities from Program line IV.
It is worth noting that proper ventilation is not only important for reducing the risk of virus contamination. The main reason for ventilation is to maintain a healthy and comfortable indoor environment by reducing nuisances caused by for example odors, or exposures to emissions from building materials.
Last update 07-05-2025
Several studies have shown that virus particles can travel through the air inside aerosols. For example, in a study by Peng et al., various Covid-19 outbreaks were modelled and compared with known pathogens such as measles and tuberculosis, the aerogenic transmission characteristics of which are well-documented. This comparison suggests that Covid-19 can also be considered an aerogenic virus.1 It has also been found that these virus particles can continue to be infectious at relatively long distances from their source (approximately 2 meters), and can thus potentially lead to infection at those distances.2 The question now, is whether the use of ventilation can reduce these infections.
By introducing clean outdoor air (ventilation), the concentration of aerosols can be diluted, reducing the risk of infection through airborne particles.3 Several studies accordingly consider ventilation to be an efficient, feasible, and acceptable intervention to reduce the risk of infection through the aerogenic route.⁴¯⁸ Implementing ventilation does not automatically reduce the number of infections throughout a building; the effect can vary significantly from one room to another.⁹ Determining the effectiveness of ventilation and (pathogenic) particle removal from the room, the so-called ‘ventilation effectiveness’ or ‘contaminant removal efficiency’, is an important parameter in this regard.10,11 The extent to which ventilation reduces the risk of infection is not a straightforward issue. Use of ventilation seems most effective in combination with other methods (masks, air purifiers).6,12,13–25 Studies have identified a number of factors affecting ventilation effectiveness:
1. Distance to the emission source
The concentration of virus particles will be highest near the source, regardless of particle size, especially in exhaled air.26 However, it is clear that current ventilation solutions will have little effect on short-distance transmission (close to the source), as airflows play a less important role at short distances, and airflows have little influence on large particles that have not yet precipitated close to the source.27–29 Social distancing is an effective way to reduce the risk of infection.³
2. Ventilation system
The effectiveness of a ventilation system depends on its design, layout, placement of inlets and outlets, ventilation speed and whether the system is properly used.7–9,30,31 In the absence of mechanical ventilation, manually opening windows and doors is important.4,7,32 Ventilation can also have negative effects. Under certain circumstances, ventilation may even exacerbate the spread of aerosols. This may occur, for instance, when air is recirculated between different rooms. Central air conditioning is an example of an air handling system where this effect may occur.⁷ Poor maintenance of systems, too, may exacerbate virus spread (due to supply of contaminated air).³² Additionally, ventilation can negatively affect the thermal and acoustic comfort of a space.³³
3. Relative humidity and temperature
Indoor relative humidity and temperature play a role in the airborne spread of certain particle sizes.⁴ At lower relative humidities (already below 80%), particles ≤ 40µm will rapidly decrease in size and weight due to evaporation, after which they can be carried much further by an air current.³⁴ For particles ≥ 80µm, this effect is negligible.³⁵ However, there are indications that virus particles in aerosols of 5-10µm are deactivated more quickly at relative humidities <45%.³⁶
Temperature differences (between inside and outside) have an effect on natural ventilation, especially in case of single-sided ventilation (a type of natural ventilation in which ventilation facilities have been inserted in only one façade). Larger temperature differentials between inside and outside increase the effectiveness of ventilation.⁶ Temperature differentials between rooms can also cause air to circulate more easily in open spaces and even intrude into rooms with closed doors. These indoor temperature differentials can be caused by the location of rooms in the building, the position of windows relative to the sun (seasonal), as well as by the presence of people.⁷
It should be noted that an expert panel consulted on the matter, considers controlling the relative humidity and temperature is considered less effective and feasible for reducing the risk of infection.⁴
4. The distribution of air in the space (mixing)
In principle, fresh outdoor air has a (very) low concentration of virus particles. Exposure to virus particles is affected both by the volume of fresh outdoor air supplied as well as by the distribution of this fresh air throughout the room. If distribution is poor/ uneven, some areas of the room may be well ventilated, while little fresh air reaches other areas. This can result in situations where the ventilation system effectively reduces particle concentration in one section of the room, while having much less of an effect in another section. 7,9,32 Whether or not this differentiated effectiveness effect occurs, is due in large part to the properties and type of ventilation system. For example, a study by Baig et al. demonstrated that integration of both inlet and exhaust of the ventilation system into the ceiling, results in a higher degree of aerosol reduction than when the exhaust is mounted low down the wall.³7
Air distribution is influenced by many other factors, including layout and furnishing of spaces, placement of ventilation openings, doors and windows, movement of people, as well as by less obvious factors such as the presence of heat pumps.7,9,37–40 Doors, screens, curtains, and even air curtains can be used to control air distribution.7,37,41 This is especially important for areas that have wide access points and are directly connected to contaminated locations.⁷ For example, a study has shown that closing curtains between beds in hospitals can reduce the flow of air enough to significantly reduce the spread of aerosols between beds.42
5. Flush time
Ventilation systems require time to reduce concentration of aerosol particles. This is called ‘flush time’. If a space is used intensively in a succession of short occupancy spans (e.g. an intensively used toilet cubicle), ventilation effectiveness will be limited.⁴³
6. Diminishing returns from higher ventilation rates
Computational research has indicated that the difference between ‘no ventilation’ and ‘some ventilation’ is most significant.44–46 In other words, if adequate ventilation is already in place, further increasing it may not contribute much. However, there is no known specific amount of ventilation that can bring the number of aerogenic infections down to an acceptable level. The study by Jia et al. suggests a volume of 10 l/s/person is necessary to create a situation comparable to an outdoor environment for short and long-distance exposure.⁴⁷ This volume is slightly higher than the ventilation level generally specified by the current Dutch Building Code (Bouwbesluit) 2012. Research by Bartels et al. has found no direct reason to adjust ventilation requirements relative to Dutch Building Code levels.⁴⁴ Discussion around the interpretation of the term 'acceptable' aside, the ventilation level required depends, among other factors, on the infectivity of the pathogen. For instance, the Omicron variant is more contagious than the Delta variant of the SARS-CoV-2 virus.
Beside the effect of ventilation, another important question is to what extent the long-distance airborne transmission contributes to the overall occurrence of infections. As yet, however, there is no unequivocal answer to this question. The item ‘What is the contribution of airborne transmission of the SARS-CoV-2 virus over long distances compared to short-distance transmission?’ addresses this further.
Literature
1. Peng Z, Rojas ALP, Kropff E, et al. Practical Indicators for Risk of Airborne Transmission in Shared Indoor Environments and Their Application to COVID-19 Outbreaks. Environ Sci Technol. 2022;56(2):1125-1137. doi:10.1021/acs.est.1c06531
2. Lednicky JA, Lauzardo M, Hugh Fan Z, et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis. 2020;(1):1-20. doi:10.1016/j.ijid.2020.09.025
3. Chen W, Qian H, Zhang N, Liu F, Liu L, Li Y. Extended short-range airborne transmission of respiratory infections. J Hazard Mater. 2022;422(June 2021):126837. doi:10.1016/j.jhazmat.2021.126837
4. de Crane D’Heysselaer S, Parisi G, Lisson M, et al. Systematic Review of the Key Factors Influencing the Indoor Airborne Spread of SARS-CoV-2. Pathogens. 2023;12(3):1-27. doi:10.3390/pathogens12030382
5. Mizukoshi A, Okumura J, Azuma K. A COVID-19 cluster analysis in an office: Assessing the long-range aerosol and fomite transmissions with infection control measures. Risk Anal. 2024;44(6):1396-1412. doi:10.1111/risa.14249
6. Villers J, Henriques A, Calarco S, et al. SARS-CoV-2 aerosol transmission in schools: The effectiveness of different interventions. Swiss Med Wkly. 2022;152(21-22):1-17. doi:10.4414/smw.2022.w30178
7. Horne J, Dunne N, Singh N, et al. Building parameters linked with indoor transmission of SARS-CoV-2. Environ Res. 2023;238(P1):117156. doi:10.1016/j.envres.2023.117156
8. Marwah M, Bambang Wispriyono, Susanna D, Kusuma A. Factors Affecting Indoor Air against the Transmission Risk of Coronavirus Disease 2019: Systematic Review and Policy Analysis. Open Access Maced J Med Sci. 2023;11(F):270-278. doi:10.3889/oamjms.2023.11160
9. Jiang C, Liu Z, Wang Y, Yao G, He J, Li S. Severity and risk to inhalation of pathogen-laden aerosol in large public spaces : Insights from fangcang shelter hospitals under multi-location release. J Hazard Mater. 2025;483(November 2024):136695. doi:10.1016/j.jhazmat.2024.136695
10. Mundt E, Mathisen HM, Nielsen P V., Moser A. REVHA Guidebook No 2 - Ventilation Effectiveness.; 2004.
11. NEN. NEN-EN-ISO 14644-3: Cleanrooms and associated controlled environments - Part 3: Test methods. Published online 2019.
12. Chen W, Qian H, Zhang N, Liu F, Liu L, Li Y. Extended short-range airborne transmission of respiratory infections. 2020;(January).
13. Liu YY, Ning Z, Chen Y, et al. Aerodynamic Characteristics and RNA Concentration of SARS-CoV-2 Aerosol in Wuhan Hospitals during COVID-19 Outbreak. bioRxiv. 2020;86(21):2020.03.08.982637. doi:10.1101/2020.03.08.982637
14. Cowling BJ, Ip DKM, Fang VJ, et al. Aerosol transmission is an important mode of influenza A virus spread. Published online 2013:1-12. doi:10.1038/ncomms2922.Aerosol
15. WHO. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br WHO. 2020;(March):10-12. doi:10.1056/NEJMoa2001316.5.
16. Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med. 2020;8(9):914-924. doi:10.1016/S2213-2600(20)30323-4
17. Huang YC, Tu HC, Kuo HY, et al. Outbreak investigation in a COVID-19 designated hospital: The combination of phylogenetic analysis and field epidemiology study suggesting airborne transmission. J Microbiol Immunol Infect. 2023;56(3):547-557. doi:10.1016/j.jmii.2023.01.003
18. Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12(11):1657-1662. doi:10.3201/eid1211.060426
19. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10). doi:10.3390/v11100940
20. Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: A systematic review. PLoS One. 2012;7(4). doi:10.1371/journal.pone.0035797
21. Li Y, Huang X, Yu ITS, Wong TW, Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;15(2):83-95. doi:10.1111/j.1600-0668.2004.00317.x
22. Grosskopf K, Mousavi E. Bioaerosols in health-care environments. ASHRAE J. 2014;56(8):22-31.
23. Lindsley WG, Blachere FM, Thewlis RE, et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One. 2010;5(11). doi:10.1371/journal.pone.0015100
24. Kutter JS, Spronken MI, Fraaij PL, Fouchier RA, Herfst S. Transmission routes of respiratory viruses among humans. Curr Opin Virol. 2018;28:142-151. doi:10.1016/j.coviro.2018.01.001
25. Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of Respiratory Viral Infections. Annu Rev Virol. Published online 2020:1-19. doi:10.1146/annurev-virology-012420-022445
26. Jones NR, Qureshi ZU, Temple RJ, Larwood JPJ, Greenhalgh T. Two metres or one : what is the evidence for physical distancing in past viruses , argue Nicholas R Jones and colleagues. Published online 2020:1-6. doi:10.1136/bmj.m3223
27. Liu L, Li Y, Nielsen P V., Wei J, Jensen RL. Short-range airborne transmission of expiratory droplets between two people. Indoor Air. 2017;27(2):452-462. doi:10.1111/ina.12314
28. Schijven J, Vermeulen LC, Swart A, et al. Exposure assessment for airborne transmission of SARS-CoV-2 via breathing , speaking , coughing and sneezing. Published online 2020.
29. Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: A commentary. BMC Infect Dis. 2019;19(1):1-9. doi:10.1186/s12879-019-3707-y
30. Chang S, Karunyasopon P, Le M, Park DY CH. Airborne migration behaviour of SARS-CoV-2 coupled with varied air distribution systems in a ventilated space. Indoor Built Environ. 32(10):2000-2019. doi:10.1177/1420326X221148084tle
31. Peerless K, Ullman E, Cummings KJ, et al. Indoor Air Quality Assessments in 10 Long-Term Care Facilities during the COVID-19 Pandemic, California, 2021–2023. J Am Med Dir Assoc. 2024;25(10):105195. doi:10.1016/j.jamda.2024.105195
32. Carrazana E, Ruiz-Gil T, Fujiyoshi S, et al. Potential airborne human pathogens: A relevant inhabitant in built environments but not considered in indoor air quality standards. Sci Total Environ. 2023;901(April):165879. doi:10.1016/j.scitotenv.2023.165879
33. de la Hoz-Torres ML, Aguilar AJ, Costa N, Arezes P, Ruiz DP, Martínez-Aires MD. Reopening higher education buildings in post-epidemic COVID-19 scenario: monitoring and assessment of indoor environmental quality after implementing ventilation protocols in Spain and Portugal. Indoor Air. 2022;32(5):282. doi:10.1111/ina.13040
34. Liu L, Wei J, Li Y, Ooi A. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air. 2017;27(1):179-190. doi:10.1111/ina.12297
35. Kompatscher K, Traversari R. TNO 2020 R11208 Rev. 1. Literatuurstudie Naar de Afstand Die Deeltjes (>5 Μm) Afleggen Bij Verschillende Respiratoire Activiteiten.; 2020.
36. Oswin HP, Haddrell AE, Otero-Fernandez M, et al. The dynamics of SARS-CoV-2 infectivity with changes in aerosol microenvironment. Proc Natl Acad Sci U S A. 2022;119(27):1-11. doi:10.1073/pnas.2200109119
37. Baig TA, Zhang M, Smith BL, King MD. Environmental Effects on Viable Virus Transport and Resuspension in Ventilation Airflow. Viruses. 2022;14(3). doi:10.3390/v14030616
38. Humphreys H, Vos M, Presterl E, Hell M. Greater attention to flexible hospital designs and ventilated clinical facilities are a pre-requisite for coping with the next airborne pandemic. Clin Microbiol Infect. 2023;29(10):1229-1231. doi:https://doi.org/10.1016/j.cmi.2023.05.014
39. Beaussier M, Vanoli E, Zadegan F, et al. Aerodynamic analysis of hospital ventilation according to seasonal variations. A simulation approach to prevent airborne viral transmission pathway during Covid-19 pandemic. Environ Int. 2022;158(April 2021). doi:10.1016/j.envint.2021.106872
40. Zhen Q, Zhang A, Huang Q, Li J, Du Y, Zhang Q. Overview of the Role of Spatial Factors in Indoor SARSCoV2 Transmission A Space-Based Framework for Assessing the Multi-Route Infection Risk. Int Journal-of-Environmental-Research-and-Public-Health. Published online 2022.
41. Park SY, Yu J, Bae S, et al. Ventilation strategies based on an aerodynamic analysis during a large-scale SARS-CoV-2 outbreak in an acute-care hospital. J Clin Virol. 2023;165(April):105502. doi:10.1016/j.jcv.2023.105502
42. Cadnum JL, Jencson AL, Alhmidi H, Zabarsky TF, Donskey CJ. Airflow Patterns in Double-Occupancy Patient Rooms May Contribute to Roommate-To-Roommate Transmission of Severe Acute Respiratory Syndrome Coronavirus 2. Clin Infect Dis. 2022;75(12):2128-2134. doi:10.1093/cid/ciac334
43. Denpetkul T, Pumkaew M, Sittipunsakda O, Leaungwutiwong P, Mongkolsuk S, Sirikanchana K. Effects of face masks and ventilation on the risk of SARS-CoV-2 respiratory transmission in public toilets: a quantitative microbial risk assessment. J Water Health. 2022;20(2):300-313. doi:10.2166/WH.2022.190
44. Bartels AA. Effect-van-Verschillende-Ventilatiehoeveelheden-Op-Aerogene-Transmissie-van-Sars-Cov-2. Risicoschatting Op Basis van Het AirCoV2-Model.; 2020.
45. Rocha-Melogno L, Crank K, Bergin MH, Gray GC, Bibby K, Deshusses MA. Quantitative risk assessment of COVID-19 aerosol transmission indoors: a mechanistic stochastic web application. Environ Technol. 2023;44(9):1201-1212. doi:10.1080/09593330.2021.1998228
46. Aho Glele LS, de Rougemont A. Non-Pharmacological Strategies and Interventions for Effective COVID-19 Control: A Narrative Review. J Clin Med. 2023;12(20). doi:10.3390/jcm12206465
47. Jia W, Wei J, Cheng P, Wang Q, Li Y. Exposure and respiratory infection risk via the short-range airborne route. Build Environ. 2022;219(April):109166. doi:10.1016/j.buildenv.2022.109166
Can air purifiers reduce infection risks?
Laboratory studies indicate the effectiveness of air purifiers in reducing the number of infectious virus particles. However, the number of studies providing practical evidence for the effectiveness of air purifiers in lowering the risk of respiratory virus infection is limited.
Air purification equipment can use various techniques to purify the air. No air purification technique or method is universally optimal; the choice of method depends on many factors such as the type of pathogen, the stability of the pathogen, whether and which ventilation strategy is applied in the space, and the location and properties of the air purifier. For the effectiveness of portable air purifiers, it is important that they are used according to the manufacturer's specifications. Because users may experience inconvenience when used according to the manufacturer's specifications (e.g., drafts, cold, noise, difficulty fitting into the space, electricity costs), portable air purifiers are often not used correctly, which reduces their effectiveness.
Given the limited evidence base, it is not recommended to trust solely to air purification methods for limiting aerogenic transmission risks. The first priority in risk mitigation should be to ensure adequate functioning of the ventilation system. Only when that has been ensured should supplementary deployment of air purification technologies be considered.
When implementing air purification, it's important to be aware of potential negative health effects from prolonged exposure to emitted by-products (such as ozone and potentially UVC).
It is worth noting that air purifiers, like ventilation, will have little or no effect on infection risk when the aerosol emitter and the receiver (person) are in close proximity.
Last update: 07-05-2025
Air purifiers are generally used to reduce indoor particle concentrations. Air purifiers filter indoor air, potentially removing or deactivating/killing microbiological contaminants (bacteria, viruses, fungi), thereby improving air quality.1,2 The SARS-CoV-2 pandemic has highlighted the importance of virus inactivation and virus particle removal.
Air purifiers use one or more technologies. Many studies are available that examine the effectiveness of purification technologies using filtration, UV, or ionization.2 Laboratory studies show that air purifiers can be effective in reducing virus particle counts.3,4 The degree of effect can vary by virus and by purification technique.4 Evidence from field studies on actual infection risk reduction is limited.5–9 Field studies do show that portable air purifiers are often used incorrectly, reducing their effectiveness. Deployment according to manufacturer specifications often causes users to experience inconvenience in terms (e.g., drafts, cold, noise, difficulty fitting into the space, electricity costs). 9–11
Evidence for the effectiveness of each technology in reducing the risk of infection is discussed below.
Filtration/HEPA
Filters can be mounted in portable air purifiers or in existing HVAC systems. Filtration studies mostly focus on effectiveness in filtering out specific particle sizes. There is little research specifically into microorganism neutralisation (specifically virus inactivation). To the extent that studies on infection risk reduction are available, these mostly focus on the use of HEPA filters. There is some evidence suggesting that air purification with HEPA filtration is effective in reducing respiratory or gastrointestinal infections, although the evidence base remains limited.4,8
UV
Scientific studies into air purification through UV irradiation do not show consensus in findings regarding effectiveness.1,2,7,12–18 A QMRA modelling study finds that a far-UVC air purifier could reduce the risk of SARS-CoV-2 infection, particularly when the concentration of the virus is moderate or low. The findings hold for a narrow bandwidth modelling scenario, and the study notes that factors such as the amount of virus emitted into the air and details of air purification system and radiation lamp configuration may influence the effectiveness of far-UVC.19 Studies on UV air purification for respiratory virus risk reduction in practical settings are very thin on the ground.18,20–24 Two such practical studies examining the effect of air purifiers on viral transmission (measured in terms of number of actual infections) found the purification technology to have no effect.18,22 A third study, conducted in care homes, where UV purifiers were installed in the existing HVAC system, found a limited but not statistically significant effect on the number of COVID-19 cases and no effect on the number of COVID-19 deaths.25 Another study in a clinical space sprayed with bacteriophage shows a reduction in infectious particles in the space through the use of a GUV purifier; this effect is stronger when the space is also ventilated.4 This study also shows that the effect of GUV varies by virus type.
The vast majority of studies on UV accordingly conclude that more research is needed to determine the mechanisms and effectiveness of air purification technology for specific microbiological contaminants.
Laboratory studies into UV irradiation exposure are mostly done using cultured microorganisms. The extent to which UV exposure inactivates or neutralises the microorganism gives some indication of its sensitivity to UV. Nebulisation of microorganisms provides a more accurate simulation of real-life effects. The effect of UV irradiation is measured by monitoring the decrease in the airborne particle count over time. This method can provide information on filtering effectiveness, but not on inactivation effectiveness. Methods to determine this inactivation effectiveness do exist, but were rarely applied in the examined literature. Neither do available studies look into the impact on effectiveness of virus particle emission from constant versus intermittent particle sources. Studies generally assume a single emission burst at the start of the experiment, which is a poor approximation of real situations.
Ionization
Ionization technologies have mainly been studied in relation to non-pathogenic particles. Studies on the effectiveness of ionization for inactivation of microbiological contaminants are scarce.20,26–28 The same holds for practical/in-situ studies.29–32 The few available studies do not draw conclusions either about the inactivation of microbiological contaminants or the effectiveness of a specific air purifier. A recent review study identified one cohort study where ionizers combined with electrostatic nano filtering demonstrated a reduced risk of infection.8
Health impact
Studies into the effects of prolonged exposure to UV or by-products released during photocatalytic oxidation (PCO) or ionizing technologies are scarce. Publications do indicate that most commercially available air purifiers emit by-products, although these can vary significantly from the manufacturers stated values. In general, it is advisable to test air purifiers thoroughly based on UV and ionization for by-products. In several European countries, this is common practice or even legally required before they can be used in public spaces. There is literature that suggests that high levels of exposure to electrons released during ionization can lead to negative health effects. There are, therefore, potential implications related to prolonged exposure to these air purification technologies.
So-called far-UVC lamps (which use a UV wavelength of 222nm) have been claimed to be as effective in inactivating viruses as lamps using the 'standard' 254 nm wavelength. There have also been claims to the effect that they produce fewer chemical by-products when used in moderately to well-ventilated spaces.33 Taking these claims into account, one study has been identified which pronounces UV lamps with a wavelength of 222nm to be safe for use when people are present.34 As yet, however, insufficient research has been conducted on the health effects of direct exposure to far-UVC. The RIVM accordingly continues to counsel against its use under these conditions.35 Further research on the release of and (prolonged) exposure to (far-)UVC, ozone, released radicals, and ionizing particles on human health has been recommended.21,36–43
Based on the limited availability of literature and the varying quality of available studies, no clear conclusions can be drawn regarding the effectiveness of the air purification technologies investigated. A recent review also shows that no air purification method is universally optimal; the choice of method depends on many factors such as the type of pathogen, the stability of the pathogen, whether and which ventilation strategy is applied in the space, and the location and properties of the air purifier.44 Based on this knowledge, it is unwise to rely solely on air purification techniques to reduce the risk of airborne transmission. Accordingly, the primary recommendation to reduce airborne infection risks in indoor environments is to ensure adequate ventilation.
Literature
1. Liu DT, Phillips KM, Speth MM, Besser G, Mueller CA, Sedaghat AR. Portable HEPA Purifiers to Eliminate Airborne SARS-CoV-2: A Systematic Review. Otolaryngol - Head Neck Surg (United States). 2022;166(4):615-622. doi:10.1177/01945998211022636
2. Mahmoudi A, Tavakoly Sany SB, Ahari Salmasi M, et al. Application of nanotechnology in air purifiers as a viable approach to protect against Corona virus. IET Nanobiotechnology. 2023;(March):289-301. doi:10.1049/nbt2.12132
3. Schulz J, Lochte VN, Blessing S, et al. Air cleaner prototype: Reduction of airborne viruses and effects of UV-C irradiation on virus concentration and RNA copy numbers considering modeled residence times and doses. Aerosol Sci Technol. Published online 2024. doi:10.1080/02786826.2024.2412633
4. Landry SA, Jamriska M, Menon VJ, et al. Ultraviolet radiation vs air filtration to mitigate virus laden aerosol in an occupied clinical room. J Hazard Mater. 2025;487(January):137211. doi:10.1016/j.jhazmat.2025.137211
5. Kompatscher K, Traversari R. Literatuurstudie Naar de Toepassing van Verschillende Luchtreinigingsmethoden Voor Inactivatie van Microbiologische Verontreinigingen.; 2022.
6. Vermeulen L, Bartels A. Meerwaarde van mobiele luchtreinigers in verminderen van transmissie van SARS-CoV-2 – een literatuurstudie. Published online September 2022. doi:10.21945/RIVM-2022-0134
7. Cadnum JL, Jencson AL, Alhmidi H, Zabarsky TF, Donskey CJ. Airflow Patterns in Double-Occupancy Patient Rooms May Contribute to Roommate-To-Roommate Transmission of Severe Acute Respiratory Syndrome Coronavirus 2. Clin Infect Dis. 2022;75(12):2128-2134. doi:10.1093/cid/ciac334
8. Brainard J, Jones NR, Swindells IC, et al. Effectiveness of filtering or decontaminating air to reduce or prevent respiratory infections: A systematic review. Prev Med (Baltim). 2023;177(July):107774. doi:10.1016/j.ypmed.2023.107774
9. AIRIAS Consortium. Gebruik van Luchtreinigers Op Scholen:Fase 1.; 2024.
10. Ebrahimifakhar A, Poursadegh M, Hu Y, Yuill DP, Luo Y. A systematic review and meta-analysis of field studies of portable air cleaners: Performance, user behavior, and by-product emissions. Sci Total Environ. 2024;912(August 2023):168786. doi:10.1016/j.scitotenv.2023.168786
11. Ding E, Giri A, Gaillard A, Bonn D, Bluyssen PM. Using mobile air cleaners in school classrooms for aerosol removal: Which, where and how. Indoor Built Environ. 2024;0(0):1-24. doi:10.1177/1420326x241267007
12. Bedell K, Buchaklian A, Perlman S. Efficacy of an automated multi-emitter whole room UV-C disinfection system against Coronaviruses MHV and MERS-CoV. Infect Control Hosp Epidemiol. 2017;37(5):598-599. doi:doi:10.1017/ice.2015.348
13. Green CF, Scarpino P V. The use of ultraviolet germicidal irradiation (UVGI) in disinfection of airborne bacteria. Environ Eng Policy. 2001;3(1):101-107. doi:10.1007/s100220100046
14. Jelden KC, Gibbs SG, Smith PW, et al. Ultraviolet (UV)-reflective paint with ultraviolet germicidal irradiation (UVGI) improves decontamination of nosocomial bacteria on hospital room surfaces. J Occup Environ Hyg. 2017;14(6):456-460. doi:10.1080/15459624.2017.1296231
15. Ko G, First MW, Burge HA. The characterization of upper-room ultraviolet germicidal irradiation in inactivating airbone microorganisms. Environ Health Perspect. 2002;110(1):95-101. doi:10.1289/ehp.0211095
16. Lin WE, Mubareka S, Guo Q, Steinhoff A, Scott JA, Savory E. Pulsed ultraviolet light decontamination of virus-laden airstreams. Aerosol Sci Technol. 2017;51(5):554-563. doi:10.1080/02786826.2017.1280128
17. Welch D, Buonanno M, Grilj V, et al. Far-UVC light: A new tool to control the spread of airborne-mediated microbial diseases. Sci Rep. 2018;8(1):1-7. doi:10.1038/s41598-018-21058-w
18. Banholzer N, Zürcher K, Jent P, et al. SARS-CoV-2 transmission with and without mask wearing or air cleaners in schools in Switzerland: A modeling study of epidemiological, environmental, and molecular data. PLoS Med. 2023;20(5):e1004226. doi:10.1371/journal.pmed.1004226
19. Blatchley ER and HC. Quantitative Microbial Risk Assessment for Quantification of the Effects of Ultraviolet Germicidal Irradiation on COVID-19 Transmission.". Environ Sci Technol 57(45) 17393-17403. Published online 2023.
20. Thornton GM, Fleck BA, Dandnayak D, Kroeker E, Zhong L, Hartling L. The impact of heating, ventilation and air conditioning (HVAC) design features on the transmission of viruses, including the 2019 novel coronavirus (COVID-19): A systematic review of humidity. PLoS One. 2022;17(10 October):1-23. doi:10.1371/journal.pone.0275654
21. Menzies D, Popa J, Hanley JA, Rand T, Milton DK. Effect of ultraviolet germicidal lights installed in office ventilation systems on workers’ health and wellbeing: Double-blind multiple crossover trial. Lancet. 2003;362(9398):1785-1791. doi:10.1016/S0140-6736(03)14897-0
22. Su C, Lau J, Gibbs SG. Student absenteeism and the comparisons of two sampling procedures for culturable bioaerosol measurement in classrooms with and without upper room ultraviolet germicidal irradiation devices. Indoor Built Environ. 2016;25(3):551-562. doi:10.1177/1420326X14562257
23. Hofbauer WK, Baßler M. Efficiency of UVC radiation as an air disinfectant in a real environment. In: Indoor Air. ; 2022.
24. Lindblad M, Tano E, Lindahl C, Huss F. Ultraviolet-C decontamination of a hospital room: Amount of UV light needed. Burns. 2020;46(4):842-849. doi:10.1016/j.burns.2019.10.004
25. Jutkowitz E, Shewmaker P, Reddy A, Braun JM, Baier RR. The Benefits of Nursing Home Air Purification on COVID-19 Outcomes: A Natural Experiment. J Am Med Dir Assoc. 2023;24(8):1151-1156. doi:10.1016/j.jamda.2023.05.026
26. Hagbom M, Nordgren J, Nybom R, Hedlund KO, Wigzell H, Svensson L. Ionizing air affects influenza virus infectivity and prevents airborne-transmission. Sci Rep. 2015;5:1-10. doi:10.1038/srep11431
27. Hyun J, Lee SG, Hwang J. Application of corona discharge-generated air ions for filtration of aerosolized virus and inactivation of filtered virus. J Aerosol Sci. 2017;107(August 2016):31-40. doi:10.1016/j.jaerosci.2017.02.004
28. Xu Y, Zheng C, Liu Z, Yan K. Electrostatic precipitation of airborne bio-aerosols. J Electrostat. 2013;71(3):204-207. doi:10.1016/j.elstat.2012.11.029
29. Bergeron V, Reboux G, Poirot JL, Laudinet N. Decreasing Airborne Contamination Levels in High-Risk Hospital Areas Using a Novel Mobile Air-Treatment Unit. Infect Control Hosp Epidemiol. 2007;28(10):1181-1186. doi:10.1086/520733
30. Meschke S, Smith BD, Yost M, et al. The effect of surface charge, negative and bipolar ionization on the deposition of airborne bacteria. J Appl Microbiol. 2009;106(4):1133-1139. doi:10.1111/j.1365-2672.2008.04078.x
31. Xia T, Lin Z, Lee EM, Melotti K, Rohde M, Clack HL. Field Operations of a Pilot Scale Packed-bed Non-thermal Plasma (NTP) Reactor Installed at a Pig Barn on a Michigan Farm to Inactivate Airborne Viruses. 2019 IEEE Ind Appl Soc Annu Meet IAS 2019. Published online 2019:7-10. doi:10.1109/IAS.2019.8912457
32. Fennelly M, O’Connor DJ, Hellebust S, et al. Effectiveness of a plasma treatment device on microbial air quality in a hospital ward, monitored by culture. J Hosp Infect. 2021;108:109-112. doi:10.1016/J.JHIN.2020.11.006
33. Peng Z, Miller SL, Jimenez JL. Model Evaluation of Secondary Chemistry due to Disinfection of Indoor Air with Germicidal Ultraviolet Lamps. Environ Sci Technol Lett. 2023;10(1):6-13. doi:10.1021/acs.estlett.2c00599
34. Pereira AR, Braga DFO, Vassal M, Gomes IB, Simões M. Ultraviolet C irradiation: A promising approach for the disinfection of public spaces? Sci Total Environ. 2023;879(December 2022). doi:10.1016/j.scitotenv.2023.163007
35. den Outer P, van Dijk A, Siegersma D, Hagens W. 2021-0050/VLH/WH Notitie UVC En Gezondheid.; 2021.
36. Medical Advisory Secretariat. Air Cleaning Technologies: An Evidence-Based Analysis. Vol 5.; 2005.
37. Jiang SY, Ma A, Ramachandran S. Negative air ions and their effects on human health and air quality improvement. Int J Mol Sci. 2018;19(10). doi:10.3390/ijms19102966
38. Cheek E, Guercio V, Shrubsole C, Dimitroulopoulou S. Portable air purification: review of impacts on indoor air quality and health. Sci Total Environ. Published online 2020:142585. doi:10.1016/j.scitotenv.2020.142585
39. Rijksinstituut voor Volksgezondheid en Milieu. Ionisatoren En Gezondheid.; 2010.
40. Blackhall K, Appleton S, Cates CJ. Ionisers for chronic asthma. Cochrane Database Syst Rev. 2012;(9). doi:10.1002/14651858.CD002986.pub2
41. Alexander DD, Bailey WH, Perez V, Mitchell ME, Su S. Air ions and respiratory function outcomes: A comprehensive review. J Negat Results Biomed. 2013;12(1):1. doi:10.1186/1477-5751-12-14
42. Liu S, Huang Q, Wu Y, et al. Metabolic linkages between indoor negative air ions, particulate matter and cardiorespiratory function: A randomized, double-blind crossover study among children. Environ Int. 2020;138(March):105663. doi:10.1016/j.envint.2020.105663
43. World Health Organization. Ultraviolet Radiation As a Hazard in the Workplace. World Heal Organ. Published online 2003.
44. Alhussain H, Ghani S, Eltai NO. Breathing Clean Air : Navigating Indoor Air Purification Techniques and Finding the Ideal Solution. Published online 2024.
How is my ventilation system performing?
What volume of air exchange is sufficient?
Within an acceptable level of estimated risk, there is no immediate reason to deviate from the requirements for new construction outlined in the 2012 ‘Bouwbesluit’ (building code). However, it may be worthwhile to consider higher standards depending on the residents' susceptibility to infection. The 2012 Bouwbesluit ventilation requirement for new-build healthcare buildings is a minimum of 6.5 dm3/s per person. For healthcare bed areas, a fresh air supply of at least 12 dm3/s per person is required.
There are no scientific publications describing the effect of supplying a specific volume of air (ventilation) on the number of potential infections transmitted in a space, or the required degree of ventilation to achieve widely accepted infection levels. However, there are various guidelines, including those from the WHO and CDC, which provide recommended volumes of fresh outdoor air.1,2 The WHO guideline recommends 60 dm3/s per person for healthcare settings. This is significantly higher than the new-build requirements in the 2012 Dutch Building Code (Bouwbesluit). The Bouwbesluit 2012 stipulates a requirement of 0.9 dm3/s per m2 of floor area, with a minimum of 7 dm3/s per person for newly built habitable rooms in residential settings. Regarding healthcare, a threshold value of 12 dm3/s per person in bed areas and 6.5 dm3/s per person in other living areas is given.3
The WHO roadmap was developed after an exploratory study of available literature and an assessment of available guidelines on building ventilation. The literature included in the study seems not to contain research specifically focused on the spread of viruses.1 Recently, The Lancet COVID-19 Commission published a report proposing Non-infectious Air Delivery Rates (NADR) to limit the risk of aerogenic respiratory infections. For schools, offices, and vehicles, a range is recommended from good (10dm3/s /person) to best (>14dm3/s /person).4
Literature
1. WHO. Roadmap to Improve and Ensure Good Indoor Ventilation in the Context of COVID-19.; 2021.
2. de Crane D’Heysselaer S, Parisi G, Lisson M, et al. Systematic Review of the Key Factors Influencing the Indoor Airborne Spread of SARS-CoV-2. Pathogens. 2023;12(3):1-27. doi:10.3390/pathogens12030382
3. Bouwbesluit Online.
4. Allen JG. Proposed Non-infectious Air Delivery Rates ( NADR ) for Reducing Exposure to Airborne Respiratory Infectious Diseases Task Force Members. Lancet. 2022;(November):1-33.
Viral transmission
How is SARS-CoV-2 transmitted?
People can be infected via three routes:
Human-to-human at close range by 'direct contact'.
By short- and long-range transmission via small droplets (aerosols) containing virus particles (the aerogenic route).
By indirect transmission via surfaces.
It is currently unclear what proportion of infections occur by which transmission route, but the risk of infection is highest through short-range transmission (direct contact and inhalation). Therefore, measures aimed at limiting short-range transmission, such as the 1.5-meter distancing guideline, are crucial. The likelihood of infection via surfaces seems to be low. Consequently, the continuous cleaning of surfaces to prevent SARS-CoV-2 infections may have a limited effect.
SARS-CoV 2 have three potential transmission routes:
Human-to-human at close range by direct deposition on mucosal surfaces.
By short- and long-range airborne transmission via inhalation (aerogenic route) of small or large droplets containing virus particles (Infectious Respiratory Particles – IRP)1
By indirect transmission via surfaces (from person to a surface to another person)1–4
The short- and long range aerogenic transmission routes are shown in Figure 1.
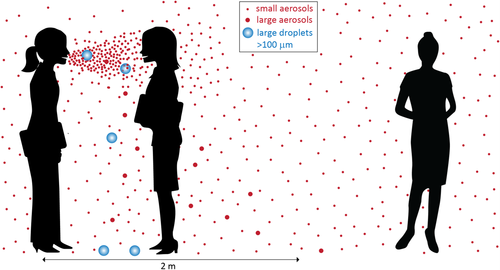 Figure 1 Transmission of SARS-CoV-2 via the air; Source: Jimenez.3
Figure 1 Transmission of SARS-CoV-2 via the air; Source: Jimenez.3
Short range transmission via direct deposition and aerogenic route
Transmission of the SARS-CoV-2 virus (and other viruses) primarily occurs at short distances through droplets and aerosols generated by various respiratory functions like breathing, talking, coughing, and sneezing.⁶⁻²⁰ The number of particles produced is correlated to the sound volume produced by the respiratory activity. Talking can produce up to ten times more virus particles in the air than just breathing.¹⁰ The amount of time between infection and droplet generation plays an important role in the quantity of virus particles present in the exhaled aerosols.21 The available literature on the topic consistently finds a higher risk of transmission at short distances involving both direct deposition and inhalation of virus particles.22–27 Well-known guidelines, such as the 1.5-meter directive from institutions like the RIVM, have been designed with this in mind.
Aerogenic route over longer distances
Beside posing a risk at short distances, aerogenic transmission (by aerosol) can also occur over longer distances.24–27 Due to their small size, these aerosols can spread through the air and thus throughout a room.28 There is an increasing amount of literature that examines the aerogenic long range transmission route.25,28 However, collecting direct evidence exclusively for the aerogenic route is very challenging. Currently, it is not possible to distinguish the degree to which different transmission routes contribute to infections. In other words, it is unknown what fraction of infections occurs via the aerogenic route, both at short and long distances. Conversely, it is also unknown what portion of infections occurs through other routes.2,6,13,15,29–39 The answer to the question 'What is the contribution of aerogenic transmission (via air) of the SARS-CoV-2 virus over long distances (1.5m or more) compared to transmission at short distances?' goes into more detail on this topic.
Indirect transmission via surfaces
The final known infection route is indirect transmission of SARS-CoV2 via surfaces. Virus-containing droplets and particles settle on surfaces and can remain infectious on a surface for several days under ideal conditions.40–42 After contact with this surface, virus particles can subsequently be transmitted from the hands to mucosal surfaces in the mouth, nose or eyes, leading to infection. The extent to which it contributes to the occurrence of infections is not known.2,7,34,43–50 The CDC considers the chance of infection with the SARS-CoV-2 virus through this last route to be small.22,23,51,52 The answer to the question 'Does the SARS-CoV-2 virus remain infectious on surfaces, and what factors influence this?' goes into more detail on this topic.
Literature
1. WHO. Global Technical Consultation Report on Proposed Terminology for Pathogens That 1. WHO. Global Technical Consultation Report on Proposed Terminology for Pathogens That Transmit through the Air.; 2024.
2. WHO. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br WHO. 2020;(March):10-12. doi:10.1056/NEJMoa2001316.5.
3. RIVM. Richtlijn COVID-19. 23 november.
4. de Crane D’Heysselaer S, Parisi G, Lisson M, et al. Systematic Review of the Key Factors Influencing the Indoor Airborne Spread of SARS-CoV-2. Pathogens. 2023;12(3):1-27. doi:10.3390/pathogens12030382
5. Jimenez JL, Marr LC, Randall K, Ewing ET, Tufekci Z, Greenhalgh T. What Were the Historical Reasons for the Resistance to Recognizing Airborne Transmission during the COVID-19 Pandemic ? SSRN Electron J. 2021;(May):1-18. doi:10.1111/ina.13070
6. Kutter JS, Spronken MI, Fraaij PL, Fouchier RA, Herfst S. Transmission routes of respiratory viruses among humans. Curr Opin Virol. 2018;28:142-151. doi:10.1016/j.coviro.2018.01.001
7. da Silvia GM. An analysis of the transmission modes of COVID-19 in light of the concepts of Indoor Air Quality. :1-12.
8. Gralton J, Tovey ER, Mclaws ML, Rawlinson WD. Respiratory virus RNA is detectable in airborne and droplet particles. J Med Virol. 2013;85(12):2151-2159. doi:10.1002/jmv.23698
9. Asadi S, Wexler AS, Cappa CD, Barreda S, Bouvier NM, Ristenpart WD. Aerosol emission and superemission during human speech increase with voice loudness. Sci Rep. 2019;9(1):1-10. doi:10.1038/s41598-019-38808-z
10. Morawska L, Johnson GR, Ristovski ZD, et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2009;40(3):256-269. doi:10.1016/j.jaerosci.2008.11.002
11. Stadnytskyi V, Bax CE, Bax A, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci U S A. 2020;117(22):19-21. doi:10.1073/pnas.2006874117
12. Qian H, Zheng X. Ventilation control for airborne transmission of human exhaled bio-aerosols in buildings. J Thorac Dis. 2018;10(Suppl 19):S2295-S2304. doi:10.21037/jtd.2018.01.24
13. Liu YY, Ning Z, Chen Y, et al. Aerodynamic Characteristics and RNA Concentration of SARS-CoV-2 Aerosol in Wuhan Hospitals during COVID-19 Outbreak. bioRxiv. 2020;86(21):2020.03.08.982637. doi:10.1101/2020.03.08.982637
14. Wei J, Li Y. Airborne spread of infectious agents in the indoor environment. Am J Infect Control. 2016;44(9):S102-S108. doi:10.1016/j.ajic.2016.06.003
15. Cowling BJ, Ip DKM, Fang VJ, et al. Aerosol transmission is an important mode of influenza A virus spread. Published online 2013:1-12. doi:10.1038/ncomms2922.Aerosol
16. Kluytmans van den Bergh MFQ, Buiting AGM, Pas SD, et al. SARS-CoV-2 infection in 86 healthcare workers in two Dutch hospitals in March 2020: a cross-sectional study with short-term follow -up. medRxiv. Published online 2020.
17. Knibbs LD, Morawska L, Bell SC. The risk of airborne influenza transmission in passenger cars. Epidemiol Infect. 2012;140(3):474-478. doi:10.1017/S0950268811000835
18. Buonanno G, Robotto A, Brizio E, et al. Link between SARS-CoV-2 emissions and airborne concentrations: Closing the gap in understanding. J Hazard Mater. 2022;428:128279. doi:10.1016/j.jhazmat.2022.128279
19. Stiti M, Castanet G, Corber A, Alden M, Berrocal E. Transition from saliva droplets to solid aerosols in the context of COVID-19 spreading. Environ Res. 2022;204(PB):112072. doi:10.1016/j.envres.2021.112072
20. Pan S, Xu C, Francis Yu CW, Liu L. Characterization and size distribution of initial droplet concentration discharged from human breathing and speaking. Indoor Built Environ. 2023;32(10):2020-2033. doi:10.1177/1420326X221110975
21. Linde KJ, Wouters IM, Kluytmans JAJW, et al. Detection of SARS-CoV-2 in Air and on Surfaces in Rooms of Infected Nursing Home Residents. Ann Work Expo Heal. 2022;XX(Xx):1-12. doi:10.1093/annweh/wxac056
22. Katona P, Kullar R, Zhang K. Bringing Transmission of SARS-CoV-2 to the Surface: Is there a Role for Fomites? Published online 2022:1-76.
23. McNeill VF. Airborne Transmission of SARS-CoV-2: Evidence and Implications for Engineering Controls. Annu Rev Chem Biomol Eng. 2022;13:123-140. doi:10.1146/annurev-chembioeng-092220-111631
24. Randall K, Ewing ET, Marr LC, Jimenez JL, Bourouiba L. How did we get here: what are droplets and aerosols and how far do they go? A historical perspective on the transmission of respiratory infectious diseases. Published online 2021. doi:10.1098/rsfs.2021.0049
25. Peng Z, Rojas ALP, Kropff E, et al. Practical Indicators for Risk of Airborne Transmission in Shared Indoor Environments and Their Application to COVID-19 Outbreaks. Environ Sci Technol. 2022;56(2):1125-1137. doi:10.1021/acs.est.1c06531
26. Tang JW, Bahnfleth WP, Bluyssen PM, et al. Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus (SARS-CoV-2) Narrative. J Hosp Infect. Published online 2021. doi:10.1016/j.jhin.2020.12.022
27. Chen W, Qian H, Zhang N, Liu F, Liu L, Li Y. Extended short-range airborne transmission of respiratory infections. 2020;(January).
28. RIVM. Aerogene verspreiding SARS-CoV-2 en ventilatiesystemen ( onderbouwing ).
29. Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: A systematic review. PLoS One. 2012;7(4). doi:10.1371/journal.pone.0035797
30. Li Y, Huang X, Yu ITS, Wong TW, Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;15(2):83-95. doi:10.1111/j.1600-0668.2004.00317.x
31. Grosskopf K, Mousavi E. Bioaerosols in health-care environments. ASHRAE J. 2014;56(8):22-31.
32. Lindsley WG, Blachere FM, Thewlis RE, et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One. 2010;5(11). doi:10.1371/journal.pone.0015100
33. Duval D, Palmer JC, Tudge I, et al. Long distance airborne transmission of SARS-CoV-2: rapid systematic review. BMJ. Published online 2022:1-14. doi:10.1136/bmj-2021-068743
34. Chen W, Zhang N, Wei J, Yen HL, Li Y. Short-range airborne route dominates exposure of respiratory infection during close contact. Build Environ. 2020;176(March):106859. doi:10.1016/j.buildenv.2020.106859
35. Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of Respiratory Viral Infections. Annu Rev Virol. Published online 2020:1-19. doi:10.1146/annurev-virology-012420-022445
36. Shiu EYC, Leung NHL, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses: Implication for infection prevention. Curr Opin Infect Dis. Published online 2019. doi:10.1097/QCO.0000000000000563
37. Buonanno G, Stabile L, Morawska L. Estimation of airborne viral emission: quanta emission rate of SARS-CoV-2 for infection risk assessment. medRxiv. Published online 2020:2020.04.12.20062828. doi:10.1101/2020.04.12.20062828
38. Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12(11):1657-1662. doi:10.3201/eid1211.060426
39. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10). doi:10.3390/v11100940
40. Doremalen N van, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. Published online 2020:1-3. doi:10.1056/NEJMc2004973
41. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246-251. doi:10.1016/j.jhin.2020.01.022
42. Chin A, Chu J, Perera M, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Infect Dis. 2020;5247(20):2020.03.15.20036673. doi:10.1016/S2666-5247(20)30003-3
43. Sandora TJ, Shih MC, Goldmann DA. Reducing absenteeism from gastrointestinal and respiratory illness in elementary school students: A randomized, controlled trial of an infection-control intervention. Pediatrics. 2008;121(6). doi:10.1542/peds.2007-2597
44. Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235-250. doi:10.1016/j.jhin.2015.08.027
45. Azor-Martínez E, Gonzalez-Jimenez Y, Seijas-Vazquez ML, et al. The impact of common infections on school absenteeism during an academic year. Am J Infect Control. 2014;42(6):632-637. doi:10.1016/j.ajic.2014.02.017
46. Snyder KM. Does Hand Hygiene Reduce Influenza Transmission? J Infect Dis. 2010;202(7):1146-1147. doi:10.1086/656144
47. Yang C. Does hand hygiene reduce SARS-CoV-2 transmission? Graefe’s Arch Clin Exp Ophthalmol. Published online 2020:5-6. doi:10.1007/s00417-020-04652-5
48. Santarpia JL, Rivera DN, Herrera V, et al. Transmission Potential of SARS-CoV-2 in Viral Shedding Observed at the University of Nebraska Medical Center. medRxiv. Published online 2020:2020.03.23.20039446. doi:10.1101/2020.03.23.20039446
49. Döhla M, Wilbring G, Schulte B, et al. SARS-CoV-2 in environmental samples of quarantined households. medRxiv. Published online June 2020:2020.05.28.20114041. doi:10.1101/2020.05.28.20114041
50. Fischer EP, Fischer MC, Grass D, Henrion I, Warren WS, Westman E. Low-cost measurement of face mask efficacy for filtering expelled droplets during speech. Sci Adv. 2020;6(36). doi:10.1126/sciadv.abd3083
51. Lewis D. COVID-19 rarely spreads through surfaces. So why are we still deep cleaning? Nature. 2021;590(7844):26-28. doi:10.1038/D41586-021-00251-4
52. Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments | CDC.
What is the contribution of airborne transmission of the SARS-CoV-2 virus over long distances compared to short-distance transmission?
Although there is still uncertainty about the extent to which long- range infections contribute to the overall indoor infection risk, the following indications hold:
Large droplets, caused by actions such as coughing, primarily pose an infection risk at close range. Wearing face masks appears to reduce this risk. Ventilation has little to no effect on transmission at short range.
Small droplets can remain airborne longer and pose an infection risk over longer distances. To limit airborne transmission over long distances, it is important to supply sufficient fresh outdoor air (low in viruses) to a space, thereby reducing the number of potentially harmful virus particles in the air. Proper ventilation thus appears to be an important measure to reduce the risk of long-distance transmission.
For more information about ventilation, see the answer to the question ‘Can ventilation reduce the risk of infection?’ and www.ventilerenzogedaan.nl. For more details on the current state of ventilation systems in Dutch long-term care facilities see the report from Program Line IV.
More and more literature is becoming available concerning SARS-CoV-2 infection through aerogenic transmission.1 Although aerogenic infection has not been conclusively demonstrated yet, indications have been found in several studies that infections through long-range (>1,5m) aerogenic transmission have occurred.2 The so-called ‘superspreading events’, especially, point in this direction.3,4
However, determining the impact of long-distance compared to shorter-distance transmission is complicated, due to insufficient information regarding the circumstances under which infections have occurred. Several modeling studies have been conducted, however, to calculate the importance of different transmission routes for certain scenarios and to determine which factors this importance depends on.5, 6, 7 Based on these modelling studies, the following factors seem to play a role:
Size & number of aerosols
The smaller the aerosol, the more important the long-distance route is, as the aerosol can remain in the air longer. The larger the proportion of small aerosols, the more important the long-distance route is. 6 Aerosol-forming activity (such as breathing, talking, coughing, and singing) characteristics affect the number and size of aerosols a person emits. 8 9 10 An infected person can exhale a much higher number of infected aerosols during speech production compared to normal breathing without speech production.9 10 Coughing mainly produces larger aerosols and droplets, a large proportion of which will precipitate quickly. Thus, coughing mainly creates a risk of infection at short distances.8 Despite these differences, most of the emitted particles are smaller than 10μm and a large percentage even smaller than 1μm.11 During sports, the number of aerosols emitted by people in the room increases.12 13 The increase is greater during endurance training such as spinning classes than during strength training.12 High-intensity (80-100% of max) endurance training is associated with the most significant increases in number of aerosols produced.
Concentration of the virus in carriers
Virus concentration in an infected person may affect the relative importance of the routes, but the mechanism is not yet entirely clear.5 7 What is clear, however, is that the higher the virus concentration in the respiratory tracks, the more virus is present in aerosols emitted, as concluded in the literature study conducted by P3 venti Program Line VI.11 Furthermore, the biochemical composition of each aerosol particle depends on its origin in the body, such as the bronchi, larynx, or mouth, and will initially be the same as that of the mucus/mucosa layer in those places. This composition influences how long viruses in the aerosols remain infectious, and thus, their ability to infect someone over longer distances. Also, virus concentration in exhaled air can vary between virus variants. For virus variants with higher virus concentrations in exhaled air (e.g., Delta and Omicron), the risk of 'superspreaders' is greater.14,15
Infection prevention measures
Infection prevention measures such as wearing face masks influence the relative contribution of the short versus the short-distance route and the indirect (via surfaces) route.7,16, 17 When wearing a face mask, larger droplets are blocked, making the short-distance route and the indirect route less important. 7
Air currents
During respiratory activities such as breathing, talking, coughing, and sneezing, aerosols (particles) and respiratory droplets of various sizes are emitted. These are then carried along by air currents in the room. Smaller particles, in particular, can travel relatively long distances, given a sufficiently strong air current.18–21 Air currents can influence the relative contribution of the routes, as aerosols can move further in the direction of the current. Air currents arise, for example, from people walking around, or through ventilation.17 Ventilation can influence the airflow by diluting and spreading aerosols.21–35 The answer to the question ‘Can ventilation reduce the risk of infections?’ goes further into this.
Rate of evaporation
Evaporation affects the size and weight of airborne particles, which in turn affects the distance particles and droplets can travel. Respiratory particles and droplets begin to evaporate as soon as they are emitted. If evaporation occurs rapidly, a droplet can cover a greater distance. If evaporation is slow, a droplet will precipitate faster, dependent on its starting size and weight. Relative humidity can have a significant effect on the distance larger particles, between 5 and 40µm in diameter at emission, can travel. Exhaled particles can reduce in size by about 30% of their original size due to evaporation.36
Other environmental factors
Ambient temperature, UV radiation, relative humidity, and CO2 concentration influence the viral stability of the SARS-CoV-2 virus. Once outside the body, the infectivity of SARS-CoV-2 slowly reduces, with certain factors possibly accelerating this process. It has been demonstrated under laboratory conditions that high temperatures and/or UV-radiation can contribute to faster inactivation of the virus in aerosols and on surfaces.37–40
Relative humidity may also have some influence on viral stability, with extreme values (both high and low) possibly causing less inactivation than moderate values.41–44 A laboratory study shows that at an average relative humidity (RH) between 40%-70%, the influenza virus is inactivated most rapidly.44 In an epidemiological study, a correlation was found between a higher number of SARS-CoV-2 deaths and lower humidity. The authors of the study explain this through mechanisms including droplet evaporation, virus stability in indoor environments, and reduced natural protection of the respiratory system in dry air.45 There are also viruses that survive better at an average relative humidity (RH 50%), indicating that the effect of humidity on virus inactivation is not straightforward and depends on other factors as well.46
The CO2 concentration can also be important.47 The infectivity of the SARS-CoV-2 virus decreases less at higher pH levels of virus-carrying aerosols or IRPs (Infectious Respiratory Particles).48 High concentrations of CO2 in the air inhibit the evaporation of bicarbonates from the IRPs, keeping the pH higher and consequently causing the virus to lose infectivity more slowly. In other words, the lower the CO2 concentration in the air, the faster inactivation occurs. This effect appears to be even stronger than that observed with changes in relative humidity.47
There is increasing evidence from modeling studies that weather conditions influence various outcome measures related to SARS-CoV-2 (infection, hospitalization, death, or superspreading potential), but the exact mechanisms remain unclear. This is partly due to the fact that different studies use diverse outcome measures.43,49 For example, a study from Hong Kong found reduced superspreading potential during cold or rainy weather. The study suggests that this may be due to fewer social activities in such weather conditions.43 In contrast, a U.S. study found that the number of hospitalizations is primarily affected by the direct impact of weather conditions on the virus or aerosols, rather than indirect effects such as changes in mobility.49
Findings from practice-based field studies are far less clear and unequivocal. Studies examining correlations between indoor air conditions and COVID-19 cases typically align with lab studies in finding that higher temperatures and mid-range relative humidity reduce the transmission risk (by accelerating inactivation)42,50,51 The exact values required are less clear. Other mechanisms may be in play, such as respiratory tract sensitivity under certain conditions.4 Furthermore, while specific temperature and relative humidity values (as yet to be determined) may reduce transmission risk in theory, this risk reduction may not be practically achievable, if these values are uncomfortable for humans or cannot be achieved in practical settings.
An additional complicating factor is that there is no consensus on the definitions of aerogenic and droplet transmission.42 Definitions used in the literature show considerable variation, and it’s not always clear from study to study which definition should be assumed.
Literature
1. RIVM. Richtlijn COVID-19. 23 november.
2. Onakpoya IJ, Heneghan CJ, Spencer EA, et al. SARS-CoV-2 and the role of close contact in transmission : a systematic review [ version 3 ; peer review : 2 approved , 1 approved with reservations , 1 not approved ]. Published online 2022.
3. Miller SL, Nazaroff WW, Jimenez JL, et al. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air. 2021;31(2):314-323. doi:10.1111/ina.12751
4. Peng Z, Rojas ALP, Kropff E, et al. Practical Indicators for Risk of Airborne Transmission in Shared Indoor Environments and Their Application to COVID-19 Outbreaks. Environ Sci Technol. 2022;56(2):1125-1137. doi:10.1021/acs.est.1c06531
5. Anand S, Krishan J, Sreekanth B, Mayya YS. A comprehensive modelling approach to estimate the transmissibility of coronavirus and its variants from infected subjects in indoor environments. Sci Rep. 2022;12(1):1-11. doi:10.1038/s41598-022-17693-z
6. Ji S, Jones RM, Lei H. Impact of respiratory aerosol size and number distribution on the relative importance of different routes in SARS-CoV-2 transmission. Risk Anal. 2024;44(5):1143-1155. doi:10.1111/risa.14227
7. Mizukoshi A, Okumura J, Azuma K. A COVID-19 cluster analysis in an office: Assessing the long-range aerosol and fomite transmissions with infection control measures. Risk Anal. 2024;44(6):1396-1412. doi:10.1111/risa.14249
8. Stiti M, Castanet G, Corber A, Alden M, Berrocal E. Transition from saliva droplets to solid aerosols in the context of COVID-19 spreading. Environ Res. 2022;204(PB):112072. doi:10.1016/j.envres.2021.112072
9. Morawska L, Johnson GR, Ristovski ZD, et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2009;40(3):256-269. doi:10.1016/j.jaerosci.2008.11.002
10. Buonanno G, Robotto A, Brizio E, et al. Link between SARS-CoV-2 emissions and airborne concentrations: Closing the gap in understanding. J Hazard Mater. 2022;428:128279. doi:10.1016/j.jhazmat.2022.128279
11. Vi P. EIGENSCHAPPEN VAN UITGEADEMDE AEROSOL DEELTJES MET SARS-COV-2 EN OMGEVING DAAROP. Published online 2024.
12. Benedikt Schumm, 1 , Marie Heiber, c , Felix Grätz , Luca Stabile, Giorgio Buonanno, , Martin Schönfelder RH, Christian J. Kähler, 2 and HW. Respiratory aerosol particle emission and simulated infection risk is greater during indoor endurance than resistance exercise. Proc Natl Acad Sci. 2023;120(9). doi:10.1073/pnas
13. Brian Cowie, MBBS, FANZCA a, b,*, Imogen Wadlow, BSc c, d, Andrew Yule, MSc e, Kristel Janssens, MSc a, i, Jason Ward, BSc d, Steve Foulkes, PhD a, i, Ruhi Humphries, PhDd, Forbes McGain, PhD f, g, Rana Dhillon, MBBS, FRACS h, André La Gerche, PhD a. Aerosol Generation During High Intensity Exercise—Implications for COVID-19 Transmission. Hear Lung Circ. 2023;32(January):67-78. https://doi.org/10.1016/j.hlc.2022.10.014%0AORIGINAL
14. Riediker M, Briceno-Ayala L, Ichihara G, et al. Higher viral load and infectivity increase risk of aerosol transmission for Delta and Omicron variants of SARS-CoV-2. Swiss Med Wkly. 2022;152(1):4-8. doi:10.4414/smw.2022.w30133
15. Sim J, Son E, Kwon M, Hwang EJ, Lee YH, Choe YJ. Risk of Severe Acute Respiratory Syndrome Coronavirus 2 Transmission in Seoul, Korea. Infect Chemother . 2024;56(2):204-212. doi:10.3947/ic.2022.0167
16. Rahn S, Köster G, Bungartz HJ. Toward unraveling airborne pathogen transmission in crowds: Parameter study for an agent-based exposure model. Saf Sci. 2024;175(July 2023):106524. doi:10.1016/j.ssci.2024.106524
17. Wu J, Weng W, Fu M, Li Y. Numerical study of transient indoor airflow and virus-laden droplet dispersion: Impact of interactive human movement. Sci Total Environ. 2023;869(October 2022):161750. doi:10.1016/j.scitotenv.2023.161750
18. Liu L, Li Y, Nielsen P V., Wei J, Jensen RL. Short-range airborne transmission of expiratory droplets between two people. Indoor Air. 2017;27(2):452-462. doi:10.1111/ina.12314
19. Schijven J, Vermeulen LC, Swart A, et al. Exposure assessment for airborne transmission of SARS-CoV-2 via breathing , speaking , coughing and sneezing. Published online 2020.
20. Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: A commentary. BMC Infect Dis. 2019;19(1):1-9. doi:10.1186/s12879-019-3707-y
21. Duval D, Palmer JC, Tudge I, et al. Long distance airborne transmission of SARS-CoV-2: rapid systematic review. BMJ. Published online 2022:1-14. doi:10.1136/bmj-2021-068743
22. Kutter JS, Spronken MI, Fraaij PL, Fouchier RA, Herfst S. Transmission routes of respiratory viruses among humans. Curr Opin Virol. 2018;28:142-151. doi:10.1016/j.coviro.2018.01.001
23. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10). doi:10.3390/v11100940
24. Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: A systematic review. PLoS One. 2012;7(4). doi:10.1371/journal.pone.0035797
25. Li Y, Huang X, Yu ITS, Wong TW, Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;15(2):83-95. doi:10.1111/j.1600-0668.2004.00317.x
26. Grosskopf K, Mousavi E. Bioaerosols in health-care environments. ASHRAE J. 2014;56(8):22-31.
27. Lindsley WG, Blachere FM, Thewlis RE, et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One. 2010;5(11). doi:10.1371/journal.pone.0015100
28. Liu YY, Ning Z, Chen Y, et al. Aerodynamic Characteristics and RNA Concentration of SARS-CoV-2 Aerosol in Wuhan Hospitals during COVID-19 Outbreak. bioRxiv. 2020;86(21):2020.03.08.982637. doi:10.1101/2020.03.08.982637
29. Cowling BJ, Ip DKM, Fang VJ, et al. Aerosol transmission is an important mode of influenza A virus spread. Published online 2013:1-12. doi:10.1038/ncomms2922.Aerosol
30. Chen W, Zhang N, Wei J, Yen HL, Li Y. Short-range airborne route dominates exposure of respiratory infection during close contact. Build Environ. 2020;176(March):106859. doi:10.1016/j.buildenv.2020.106859
31. Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of Respiratory Viral Infections. Annu Rev Virol. Published online 2020:1-19. doi:10.1146/annurev-virology-012420-022445
32. WHO. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br WHO. 2020;(March):10-12. doi:10.1056/NEJMoa2001316.5.
33. Shiu EYC, Leung NHL, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses: Implication for infection prevention. Curr Opin Infect Dis. Published online 2019. doi:10.1097/QCO.0000000000000563
34. Buonanno G, Stabile L, Morawska L. Estimation of airborne viral emission: quanta emission rate of SARS-CoV-2 for infection risk assessment. medRxiv. Published online 2020:2020.04.12.20062828. doi:10.1101/2020.04.12.20062828
35. Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12(11):1657-1662. doi:10.3201/eid1211.060426
36. Mikhailov E, Vlasenko S, Niessner R, Pöschl U. Interaction of aerosol particles composed of protein and salts with water vapor: hygroscopic growth and microstructural rearrangement. Atmos Chem Phys Discuss. 2003;3(5):4755-4832. doi:10.5194/acpd-3-4755-2003
37. Schuit M, Ratnesar-Shumate S, Yolitz J, et al. Airborne SARS-CoV-2 is rapidly inactivated by simulated sunlight. J Infect Dis. 2020;222(4):564-571. doi:10.1093/infdis/jiaa334
38. Dabisch P, Schuit M, Herzog A, et al. The influence of temperature, humidity, and simulated sunlight on the infectivity of SARS-CoV-2 in aerosols. Aerosol Sci Technol. 2021;55(2):142-153. doi:10.1080/02786826.2020.1829536
39. Doremalen N van, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. Published online 2020:1-3. doi:10.1056/NEJMc2004973
40. Fears AC, Klimstra WB, Duprex P, et al. Comparative dynamic aerosol efficiencies of three emergent coronaviruses and the unusual persistence of SARS-CoV-2 in aerosol suspensions. medRxiv. 2020;2:2020.04.13.20063784. doi:10.1101/2020.04.13.20063784
41. Oswin HP, Haddrell AE, Otero-Fernandez M, et al. The dynamics of SARS-CoV-2 infectivity with changes in aerosol microenvironment. Proc Natl Acad Sci U S A. 2022;119(27):1-11. doi:10.1073/pnas.2200109119
42. de Crane D’Heysselaer S, Parisi G, Lisson M, et al. Systematic Review of the Key Factors Influencing the Indoor Airborne Spread of SARS-CoV-2. Pathogens. 2023;12(3):1-27. doi:10.3390/pathogens12030382
43. Chong KC, Zhao S, Hung CT, et al. Association between meteorological variations and the superspreading potential of SARS-CoV-2 infections. Environ Int. 2024;188(January):108762. doi:10.1016/j.envint.2024.108762
44. Motos G, Schaub A, David SC, et al. Dependence of aerosol-borne influenza A virus infectivity on relative humidity and aerosol composition. 2024;(October):1-13. doi:10.3389/fmicb.2024.1484992
45. Keetels GH, Godderis L, van de Wiel BJH. Associative evidence for the potential of humidification as a non-pharmaceutical intervention for influenza and SARS-CoV-2 transmission. J Expo Sci Environ Epidemiol. 2022;32(5):720-726. doi:10.1038/s41370-022-00472-3
46. Kim, Su Jung et al. Temperature and humidity influences on inactivation kinetics of enteric viruses on surfaces. Environ Sci Technol. 2012;13303-1331.
47. Haddrell A, Oswin H, Otero-fernandez M, et al. Ambient Carbon Dioxide Concentration Correlates with SARS-CoV-2 Aerostability and Infection Risk. Preprint. Published online 2023:1-22.
48. WHO. Global Technical Consultation Report on Proposed Terminology for Pathogens That Transmit through the Air.; 2024.
49. Grover EN, Buchwald AG, Ghosh D, Carlton EJ. Does behavior mediate the effect of weather on SARS-CoV-2 transmission? evidence from cell-phone data. PLoS One. 2024;19(6 June):1-24. doi:10.1371/journal.pone.0305323
50. Verheyen CA, Bourouiba L. Associations between indoor relative humidity and global COVID-19 outcomes. J R Soc Interface. 2022;19(196). doi:10.1098/rsif.2021.0865
51. Horne J, Dunne N, Singh N, et al. Building parameters linked with indoor transmission of SARS-CoV-2. Environ Res. 2023;238(P1):117156. doi:10.1016/j.envres.2023.117156
Do many SARS-CoV-2 infections occur in the healthcare sector?
The hospital and long-term healthcare systems are at an increased risk regarding respiratory viruses. An important reason is that due to old age, disability or sickness, hospital patients, and care home residents and clients are more likely to have an impaired immune system or comorbidities.1 As a result, a respiratory disease is more likely to spread and have serious consequences. Gilbert et al. has shown that while only 7% of people infected with COVID-19 in Australia live in care homes, this group accounted for 75% of Australian COVID-19 related deaths.2
This risk of infection is further increased in healthcare settings as groups of people live or reside in close proximity to one another. This applies even more to care homes than to hospitals, as is shown in a Japanese study.3 Another study has shown that in healthcare settings, infections are more often clustered in groups of 5 or more compared to other settings including household, restaurant, and office. In this study, such ‘clusters’ refer to a group of infected people (with a positive SARS-C0V-2 test) who had close contact with the same confirmed case(s), if the onset and confirmation of cases were fewer than 14 days apart.4
Staff in care home facilities can play a major role in spreading viruses as they are regularly in close contact with others (residents and other staff members) as part of their daily routine, according to studies from Spain and the US.5,6 During quarantines, patients, clients and residents rarely leave the healthcare facility’s premises, so there is a small chance for them to be infected elsewhere. Members of staff on the other hand, are often also active outside the facility, and are therefore more likely to introduce a pathogen into the healthcare facility. This risk is heightened for members of staff working at multiple facilities.7 As of yet, it is unknown whether members of staff are infected mostly within or outside of healthcare settings. This seems to be strongly case dependent.7–9
Various characteristics of long-term care facilities have been associated with lower infection rates.10–12 In a study conducted in English facilities, those with fewer bedrooms, better ventilation, lower bedroom temperatures, and facilities to cohort infected residents, such as on different floors, appeared more effective at preventing the spread of infections.11 A Canadian study associated facilities that performed poorly on standardized infection prevention measures with an increase in the severity of SARS-CoV-2 outbreaks.10 A U.S. study found that ventilation systems in long-term care facilities are often not used properly, which limits their effectiveness in preventing virus spread.12 This finding is also confirmed for Dutch long-term care facilities.13 For more information on the use of ventilation systems in Dutch long-term care facilities, see the report from Program Line IV.
Literature
1. Sim J, Son E, Kwon M, Hwang EJ, Lee YH, Choe YJ. Risk of Severe Acute Respiratory Syndrome Coronavirus 2 Transmission in Seoul, Korea. Infect Chemother . 2024;56(2):204-212. doi:10.3947/ic.2022.0167
2. Gilbert GL. Aged care residents — and everybody else — would benefit from better control of COVID-19 transmission. Med J Aust. 2023;218(4):166-167. doi:10.5694/mja2.51843
3. Ueda M, Hayashi K, Nishiura H. Identifying High-Risk Events for COVID-19 Transmission: Estimating the Risk of Clustering Using Nationwide Data. Viruses. 2023;15(2). doi:10.3390/v15020456
4. Imamura T, Watanabe A, Serizawa Y, et al. Transmission of COVID-19 in Nightlife, Household, and Health Care Settings in Tokyo, Japan, in 2020. JAMA Netw Open. 2023;6(2):E230589. doi:10.1001/jamanetworkopen.2023.0589
5. Adams C, Chamberlain A, Wang Y, et al. The Role of Staff in Transmission of SARS-CoV-2 in Long-term Care Facilities. Epidemiology. 2022;33(5):669-677. doi:10.1097/EDE.0000000000001510
6. Montero-Moraga JM, Buron A, Sala M, et al. Impact and management of COVID-19 among healthcare workers in two acute care hospitals and two associated long-term care centres in Barcelona, Spain. NBER Work Pap. Published online 2013:89. http://www.nber.org/papers/w16019
7. Sullivan SG, Sadewo GRP, Brotherton JM, et al. The spread of coronavirus disease 2019 (COVID-19) via staff work and household networks in residential aged-care services in Victoria, Australia, May-October 2020. Infect Control Hosp Epidemiol. 2023;44(8):1334-1341. doi:10.1017/ice.2022.243
8. Cheng VCC, Wong SC, Tong DWK, et al. Multipronged infection control strategy to achieve zero nosocomial coronavirus disease 2019 (COVID-19) cases among Hong Kong healthcare workers in the first 300 days of the pandemic. Infect Control Hosp Epidemiol. 2022;43(3):334-343. doi:10.1017/ice.2021.119
9. Billock RM, Groenewold MR, Sweeney MH, de Perio MA, Gaughan DM, Luckhaupt SE. Reported exposure trends among healthcare personnel COVID-19 cases, USA, March 2020–March 2021. Am J Infect Control. 2022;50(5):548-554. doi:10.1016/j.ajic.2022.01.007
10. Vijh R, Ng CH, Shirmaleki M, Bharmal A. Factors associated with transmission of COVID-19 in long-term care facility outbreaks. J Hosp Infect. 2022;119:118-125. doi:10.1016/j.jhin.2021.11.008
11. Krutikov M, Stirrup O, Fuller C, et al. Built Environment and SARS-CoV-2 Transmission in Long-Term Care Facilities: Cross-Sectional Survey and Data Linkage. J Am Med Dir Assoc. 2024;25(2):304-313.e11. doi:10.1016/j.jamda.2023.10.027
12. Peerless K, Ullman E, Cummings KJ, et al. Indoor Air Quality Assessments in 10 Long-Term Care Facilities during the COVID-19 Pandemic, California, 2021–2023. J Am Med Dir Assoc. 2024;25(10):105195. doi:10.1016/j.jamda.2024.105195
13. Weersink AMS, Salemink GAM, Struck C. Technische en gebruiksinventarisatie ventilatiesystemen in bestaande gebouwen voor langdurige zorg , op basis van een taxonomie en vragenlijst. 2024;(april):122. https://p3venti.nl/assets/rapportages/p3venti_rapportage_saxion_inventarisatie-gezondheidszorggebouwen.pdf
Is there an increased risk of SARS-CoV-2 infections within sports facilities?
Fitness rooms are public indoor spaces where relatively more SARS-CoV-2 infections occur compared to other indoor spaces such as workplaces, restaurants, and shopping centers, according to a systematic review and meta-analysis that examined the number of new infections in various indoor spaces.1 The study indicates that this is due to high-intensity exercise (resulting in more aerosol formation), not wearing face masks, prolonged close contact, and insufficient ventilation. The study advises taking measures to prevent the spread in these indoor spaces, such as good ventilation and a maximum number of athletes in the room at the same time. They did not test the effect of these measures.
Other studies also show that during exercise, the number of aerosols emitted by people into the room increases.2,3 During high-intensity training (80-100% of the maximum), the number increases the most. The increase is greater during cardio workouts like spinning classes than during strength training.3
Literature
1. Francis MR, Gidado S, Nuorti JP. The Risk of SARS-CoV-2 Transmission in Community Indoor Settings : A Systematic Review and Meta-analysis. J Infect Dis. 2024;230(4):824-836. doi:10.1093/infdis/jiae261
2. Brian Cowie, MBBS, FANZCA a, b,*, Imogen Wadlow, BSc c, d, Andrew Yule, MSc e, Kristel Janssens, MSc a, i, Jason Ward, BSc d, Steve Foulkes, PhD a, i, Ruhi Humphries, PhDd, Forbes McGain, PhD f, g, Rana Dhillon, MBBS, FRACS h, André La Gerche, PhD a. Aerosol Generation During High Intensity Exercise—Implications for COVID-19 Transmission. Hear Lung Circ. 2023;32(January):67-78. https://doi.org/10.1016/j.hlc.2022.10.014%0AORIGINAL
3. Benedikt Schumm, 1 , Marie Heiber, c , Felix Grätz , Luca Stabile, Giorgio Buonanno, , Martin Schönfelder RH, Christian J. Kähler, 2 and HW. Respiratory aerosol particle emission and simulated infection risk is greater during indoor endurance than resistance exercise. Proc Natl Acad Sci. 2023;120(9). doi:10.1073/pnas
Does the SARS-CoV-2 virus remain infectious on surfaces, and what factors influence this?
According to lab studies, SARS-CoV-2 can remain infectious for hours to days after being deposited on a surface. It can then become airborne again from this surface (resuspension).1–4 Through hand contact, virus particles can then be transferred to the mouth, nose, or eyes (mucosal surfaces) which can lead to infection. The type of surface on which the virus is present may affect its inactivation and the likelihood of transmission.4,5 Generally, SARS-CoV-2 was found less frequently on porous surfaces such as cotton or paper than on non-porous surfaces like metal. This could mean that the virus inactivates faster on porous surfaces (due to different conditions within the porous surface compared to the air) or that it was simply physically more challenging to take samples from porous surfaces.4
Additionally, high temperature and UV intensity/duration also accelerate the inactivation of the SARS-CoV-2 virus on surfaces.4–6 For example, sunlight can inactivate the virus on outdoor surfaces within 20 minutes.7 In some literature, it has been shown that exposure to UV-C can inactivate the SARS-CoV-2 virus on surfaces.8–11 UV-C light could therefore be used for disinfecting surfaces. However, it should be noted that UV-C is harmful to humans, so such disinfection should only be carried out when no people are present. Furthermore, the effectiveness of this virus inactivation method is also highly dependent on the UV-C exposure dose and duration.
On the other hand, the effect of humidity on the inactivation of the SARS-CoV-2 virus on surfaces, is not as clear-cut.6
By now, the general consensus is that transmission via surfaces likely plays a minor role compared to other transmission routes: short-range direct deposition and the airborne route.12,13 The answer to the question 'What is the contribution of airborne transmission of the SARS-CoV-2 virus over long distances compared to short-distance transmission?' goes into more detail on this topic.
Literature
1. Doremalen N van, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. Published online 2020:1-3. doi:10.1056/NEJMc2004973
2. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246-251. doi:10.1016/j.jhin.2020.01.022
3. Chin A, Chu J, Perera M, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Infect Dis. 2020;5247(20):2020.03.15.20036673. doi:10.1016/S2666-5247(20)30003-3
4. Horne J, Dunne N, Singh N, et al. Building parameters linked with indoor transmission of SARS-CoV-2. Environ Res. 2023;238(P1):117156. doi:10.1016/j.envres.2023.117156
5. Cox J, Christensen B, Burton N, et al. Transmission of SARS-CoV-2 in the Workplace: Key Findings from a Rapid Review of the Literature. Vol 57.; 2024. doi:10.1080/02786826.2023.2166394.Transmission
6. Geng Y, Wang Y. Stability and transmissibility of SARS-CoV-2 in the environment. J Med Virol. 2023;95(1). doi:10.1002/jmv.28103
7. Ratnesar-shumate S, Williams G, Green B, et al. OUP accepted manuscript. J Infect Dis. 2020;(52281):1-9. doi:10.1093/infdis/jiaa274
8. Kitagawa H, Nomura T, Nazmul T, et al. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am J Infect Control. 2020;000:17-19. doi:10.1016/j.ajic.2020.08.022
9. Lindblad M, Tano E, Lindahl C, Huss F. Ultraviolet-C decontamination of a hospital room: Amount of UV light needed. Burns. 2020;46(4):842-849. doi:10.1016/j.burns.2019.10.004
10. Welch D, Buonanno M, Grilj V, et al. Far-UVC light: A new tool to control the spread of airborne-mediated microbial diseases. Sci Rep. 2018;8(1):1-7. doi:10.1038/s41598-018-21058-w
11. Kompatscher K, Traversari R. Literatuurstudie Naar de Toepassing van Verschillende Luchtreinigingsmethoden Voor Inactivatie van Microbiologische Verontreinigingen.; 2022.
12. Katona P, Kullar R, Zhang K. Bringing Transmission of SARS-CoV-2 to the Surface: Is there a Role for Fomites? Published online 2022:1-76.
13. McNeill VF. Airborne Transmission of SARS-CoV-2: Evidence and Implications for Engineering Controls. Annu Rev Chem Biomol Eng. 2022;13:123-140. doi:10.1146/annurev-chembioeng-092220-111631
What is the effect of environmental conditions on the number of SARS-CoV-2 cases?
Field studies that have examined the effect of outdoor environments on the number of SARS-CoV-2 cases and mortality at the population level mainly focus on weather conditions such as temperature, humidity, precipitation, and wind. The results of these studies are inconclusive and sometimes even seem to draw contradictory conclusions. For example, in some studies, wind is the most significant factor, while in others, wind plays no role, and temperature is the most important. In practice, many more factors influence the number of cases or mortality, such as the geographical environment (e.g., altitude and latitude), demographic characteristics, other environmental features like air quality, and human behaviour under different weather conditions and in different countries. Therefore, these complexities make causal relationships difficult to investigate, and the aforementioned studies only offer possible correlations.1–8
Some studies do examine interaction effects between various environmental factors such as temperature, relative humidity, absolute humidity, and multiple weather elements (where the effect of one element on COVID-19 cases depends on another element), which could further explain the inconclusive and contradictory results.5,9
Literature
1. Aboura S. The influence of climate factors and government interventions on the Covid-19 pandemic: Evidence from 134 countries. Environ Res. 2022;208(January):112484. doi:10.1016/j.envres.2021.112484
2. Al-Khateeb MS, Abdulla FA, Al-Delaimy WK. Long-term spatiotemporal analysis of the climate related impact on the transmission rate of COVID-19. Environ Res. 2023;236(P1):116741. doi:10.1016/j.envres.2023.116741
3. Faruk MO, Rana MS, Jannat SN, Khanam Lisa F, Rahman MS. Impact of environmental factors on COVID-19 transmission: spatial variations in the world. Int J Environ Health Res. 2023;33(9):864-880. doi:10.1080/09603123.2022.2063264
4. Ravelli E, Gonzales Martinez R. Environmental risk factors of airborne viral transmission: Humidity, Influenza and SARS-CoV-2 in the Netherlands. Spat Spatiotemporal Epidemiol. 2022;41:100432. doi:10.1016/j.sste.2021.100432
5. Tateo F, Fiorino S, Peruzzo L, et al. Effects of environmental parameters and their interactions on the spreading of SARS-CoV-2 in North Italy under different social restrictions. A new approach based on multivariate analysis. Environ Res. 2022;210(January):112921. doi:10.1016/j.envres.2022.112921
6. Song Q, Qian G, Mi Y, Zhu J, Cao C. Synergistic influence of air temperature and vaccination on COVID-19 transmission and mortality in 146 countries or regions. Environ Res. 2022;215(P1):114229. doi:10.1016/j.envres.2022.114229
7. Song P, Han H, Feng H, et al. High altitude Relieves transmission risks of COVID-19 through meteorological and environmental factors: Evidence from China. Environ Res. 2022;212(PB):113214. doi:10.1016/j.envres.2022.113214
8. Lednicky JA, Lauzardo M, Hugh Fan Z, et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis. 2020;(1):1-20. doi:10.1016/j.ijid.2020.09.025
9. Geng Y, Wang Y. Stability and transmissibility of SARS-CoV-2 in the environment. J Med Virol. 2023;95(1). doi:10.1002/jmv.28103
What can I do to reduce virus transmission?
How can I best use the ventilation system?
For the proper use of a ventilation system, please refer to https://www.ventilerenzogedaan.nl [1]. This website provides 5 basic tips on how best to handle a ventilation system to ensure proper use of the ventilation facilities. Additionally, users can create a so-called ‘ventilatiekaart’ or ventilation guide, which shows information on how to best use the ventilation facilities present in the room. By placing this ventilation guide by the entrance to the room, users can quickly understand the correct use of the facilities. The publication ‘Ventilatie in relatie tot COVID-19 en een goede binnenluchtkwaliteit’ can be used to test the amount of ventilation in more detail [2].
[1] Heumen S van, Weerdt C van der, Jacobs P, Traversari R, Hinkema M. Achtergronddocument Handreiking Ventileren Zo Gedaan. Delft, The Netherlands; 2022.
[2] Binnenklimaattechniek. Ventilatie in Relatie Tot COVID-19 En Een Goede Binnenluchtkwaliteit; 2021.
Leadership:
Contact: penvoerder-p3venti@tno.nl









